This experiment aims to extract the DNA from the cheek cells and determine the length of the DNA part of the unknown electrician. DNA was extracted by Swecardi in the mouth, followed by confusion with detergents, pineapple juice and ezopropil alcohol, which wheel the DNA. The extracted DNA was imagined in the alcohol and solution interface. For electric jelly, DNA samples, including the dye mixture, unknown DNA, and DNA signs, were loaded in Agharose gel, and an electrical field was applied to separate the fragments according to the size. The distance deported by the permitted DNA signs to estimate the length of the unknown DNA part. The results showed that the unknown DNA part was about 2.7 kg (kilograms), based on the semi -logical standard. This experiment has successfully demonstrated DNA and electrical extraction, providing insight into the molecular biology techniques for DNA analysis.
Electric gel separates DNA, RNA or proteins depending on the size (Hames et.al, 1990). Each DNA molecule is a double snail consisting of complementary nucleotides that are collected by hydrogen bonds between the basic pairs (G) -cystosine (C) and Edinin (A) -Thymit (T). DNA consists of a spine that negatively charged the migration to the positive pole under the electric field (alberts et., 2002). Tribes such as ethdium bromide and SmartGlow before stain (non -cancer of origin) depicting DNA by Easter under UV light. The molecules move across the gel pores at rates that are inversely proportional to their length; Smaller fragments migrate faster (Mika and others, 2024).
The gels are used in the first place in the electrician gel, as an electrical field is applied to separate the vital molecules based on their size and charging. The gel matrix works as a molecule, allowing smaller molecules to migrate faster than large molecules. Gelgrose is a gel -like substance derived from Ajar. The number of sliced obtained from red algae. It is widely used in the molecular biology of the separation and analysis of nucleic acids (DNA and RNA) and proteins. The concentration of gel affects the size of the pores and energy solution, and deportation distances can be drawn against the logarithm on the molecular length. DNA or stairs, which contain known sizes of well -known fragments, allow the size of the unknown samples by comparing the deportation distances. By creating a standard curve of well -known molecules, the lengths of the unknown DNA can be calculated, usually expressed in kilobase (KB) or base pairs (Lee et. Al, 2012).
The goal of this experiment is to measure the length of the unknown DNA by analyzing its electrical deportation compared to standard DNA fragments of the known lengths.
If the DNA part is unknown as a similar migrant to a part of the standard DNA with a well -known length, its size can be estimated based on that comparison.
DNA extract from cheek cells
DNA extract from the cheek cells involves reducing 5 ml of Gatorade in its mouth for two minutes, then transferring the solution to the test tube. After adding 2 ml of dishwashing detergents and tubes to mix them, 2 ml of pineapple juice was added to the solution. The tubes were turned to mix. Next, 2 ml of the ice cold elzopropyl alcohol was added, and the tube was left for 10 minutes for DNA sedimentation (Mika et al., 2024).
Preparing the Great Agharz
For the next experiment, the Agruise gels are prepared in advance and stored with a gray plastic comb in place. This comb was carefully removed after the gel has been established and saved for reuse. Each student is appointed as a specific gel and electricity unit, with the room design to accommodate one gel. When loading samples, the final wells were advised to prevent pollution, and students were encouraged to load two samples of each type of DNA if desired.
Agarose electric gel
Each gel is loaded with three types of samples: dye mixture, unknown DNA, and DNA mark, with each well carrying about 25 تر l from the sample.
DNA samples are prepared and frozen before the experiment. To download the samples, Micropipeette has been used with an eliminated pipette. The edge was immersed in the sample solution, and by pressing the thumb button, 25 تر l from the sample was drawn at the tip of the tubes. Caution is exercised to check any air bubbles and expel them carefully before the PIPET tip is directed to the flooded well of the gel. Each well with a capacity of 25 ومر رار molly was filled without excessive preparedness, and a new pipette has been used for each sample to avoid mutual pollution. After downloading, the sample bottles were returned to the freezer.
Once the DNA samples are loaded, the gels were placed in the electrical room with the closest wells to the black (negative) pole, ensuring that the DNA will migrate to the red electrode (positive). The temporary store solution (0.04 M TRIS-ACETATE EDTA, the pH 8.0) has been prepared in advance, and approximately 200 ml of this cold temporary warehouse was added to completely cover the gels, eliminating any besieged air bubbles. The orange cap was gently placed on top, and adjusted as necessary.
A clear 8 cm ruler was placed on the cap, aligned with wells to visually monitor the immigration of DNA. Then the gel electrical unit was connected to a power source, and the settings were set to run at 100 volts for 35 minutes. The timer was set, and the start button was pressed once all the parameters are confirmed. Progress in DNA migration was observed by running the blue LED light below the room, which lit the gangs while moving away from the wells.
The electricity continued until the dye mixture migrated to 2-3 mm of the end of the gel. After running, the unit was turned off, and the cables were separated. The gels were imagined under the blue LED light, and the photos were taken using a black photography box with an orange filter registered on the cap. Caution is taken to align the camera with the ruler for a precise measuring scope.
Data analysis
After filming, the distance that was deported was measured by the MM DNA part, using the ruler. The results were recorded in a specific schedule for analysis. This distance was drawn on a semi -Logaretian graph against the known sizes of DNA signs to estimate the length of the unknown part of kilobase (KB). The distance deported by the unknown DNA part of the well to the front edge of the band was measured. Using the standard curve, the corresponding value on the X axis of the graph, which is the length of the unknown DNA part (Mika et al., 2024).
A simple protocol
DNA extract from cheek cells:
- Swish 5 ml of Gatorade in the mouth for two minutes.
- Transfer the solution to the test tube.
- Add 2 ml of dishwashing detergents and a spiral to mix them.
- Add 2 ml of pineapple juice and tube mind to mix.
- Gently add 2 ml of cold egopropyl alcohol.
- Leave the tube for 10 minutes to precipitate DNA.
Agarose Gel Electrophoresis:
- Prepare the gels of Agharose in advance, and store them with a gray plastic comb.
- Carefully remove the comb after hardening the gel for reuse.
- Set the specified gels and Electrophoresis units for each student.
- Skip the final wells to avoid pollution when loading samples.
- Download three types of samples in wells: dye mixture, unknown DNA, and DNA sign (25 تر l per well).
- Prepare and freeze DNA samples before the experiment.
- Use micropipeette with an absorbed absorbent tip to load samples in wells.
- Ensure that there are no air bubbles at an absorbent party and use new advice for each sample.
- Make sure to put the gel in the electric room with wells near the black (negative).
- Preparation and cold 200 ml of the temporary store solution (0.04 M TRIS-Actate Edta, PH 8.0).
- Add the temporary store to cover the gels completely, and remove air bubbles.
- Place the orange cap, and set it if necessary.
- Use a clear 8 -cm ruler to visually monitor DNA deportation, aligned with wells.
- Set the electric unit over 100 volts for 35 minutes.
- Watch the DNA migration using the blue LED light below the room.
- Keep the electrons until the dye mixture is 2-3 mm from the end of the gel.
- After running, turn off the unit and separate the cables.
- Imagine gels under the blue LED light and take pictures using a black photography box with an orange filter.
- Align the camera with the ruler for an accurate measurement of domains.
Data analysis:
- Measure the distance deported by the MM DNA part with the ruler.
- Record the measurements in an analysis schedule.
- Draw the deportation distance on a semi -Logographic graph against the well -known DNA sizes.
- Estimate the length of the DNA part of unknown cobas (KB) using the standard curve.
results
The DNA extracted from the cheek cells successfully deposited and became visible at the alcohol interface and solution in the test tube.
The distance that was deported was measured by the DNA part. The distances were drawn in a semi -reddish graph.
| DNA The brand’s shrapnel number | DNA The length of part of the maker (KBP) | DNA The shrapnel of the mark (mm) has been deported |
| 1 | 23.13 | 18 |
| 2 | 9.41 | 25 |
| 3 | 6.68 | 30 |
| 4 | 4.36 | 38 |
| 5 | 2.32 | 58 |
| 6 | 2.03 | 63 |
Table 1: The length of the part of the DNA tag
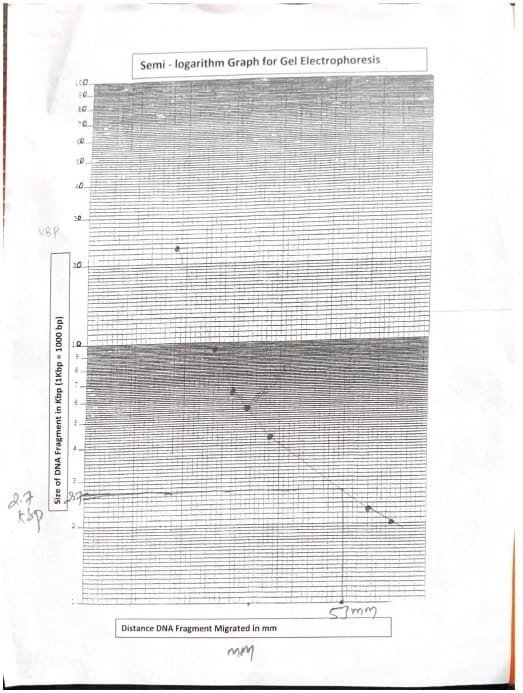
The distance that was deported by an unknown DNA was measured and the length of the part is determined by comparing the value of the mark in semi -surface paper.
| An unknown DNA part (mm) has been deported | The length of the DNA fragment is unknown (KBP) |
| 53mm | 2.7 KB |
Table 2: Determine the length of the DNA fragment is unknown
discussion
DNA was extracted from the cheek cells by adding different solutions, which facilitated the precipitation and made it visible at the alcohol interface and solution in the test tube. DNA molecules are very small so that they cannot be imagined and can only be seen using an electronic microscope, but the clump makes it visible (MIKA et., 2024).
The electric gel experience has effectively showed the separation of DNA fragments on the basis of size, using Agharose gel as a means of (Hames Et. Al, 1990). The temporary store of Edta-Actate 0.04 M TRIS at the pH is 8.0 DNA negatively charged towards the positive pole (Alberts et. Al, 2002). The standard curve built from DNA signs known to estimate the length of the unknown part at about 2.7 kg (KB).
The distinguished separation of the dye mixture confirmed the safety of the electricity process, indicating that the gel was working properly. The visualization under the blue light enabled the clear photography of the DNA, and the accurate alignment with the ruler ensures accurate measurements from a distance.
In general, this experiment has successfully clarified the principles of gel’s electric journey, while emphasizing the importance of preparing the clean sample and experimental conditions for reliable results. Future studies can achieve different Agruise concentrations or include additional controls to enhance the analysis.
Reference
- Hames, BD, & Rickwood, D. (1990). Nuclear acid gel: a practical approach. Oxford University Press.
- Alberts B, Johnson A, Lewis J, et al. The molecular biology of the cell. Fourth edition. New York: Garland Science; 2002. DNA structure and function. Available from: https://www.ncbi.nlm.nih.gov/books/nbk26821/
- Mika, TA, Klein, RJ, Bullerjahn, AE, Connour, RL, Swimmer, LM, White, R.
- E., GOSSES, MW, Carter, TE, Andrews, AM, Maier, JL, & Sidiq, F. (EDS.). (2024). Anatomy and biotechnology 211 laboratory guide (Third Edition). Owens Community College.
- Lee, PY, Costumbrado, J., HSU, CY, & Kim, YH (2012). Agarose electric gel to separate DNA fragments. Visual Experience Magazine: Jouf(62), 3923. Https://doi.org/10.3791/3923


